Have you ever taken a medication that gave you a reaction no one else seemed to get? Maybe you got dizzy from a common painkiller, or broke out in a rash after a drug your friend took without issue. It’s not just bad luck. For many people, the reason lies in their DNA.
Some of us are born with genetic variations that make our bodies process drugs differently - sometimes dangerously so. These differences aren’t rare. They’re built into our biology, and they can turn a routine prescription into a life-threatening event. The science behind this is called pharmacogenomics, and it’s reshaping how doctors choose medications - if they know how to use it.
Why Your Genes Control How Drugs Affect You
Your body doesn’t treat every drug the same way. What happens after you swallow a pill depends on three things: how fast your body absorbs it, how quickly it breaks it down, and how your cells respond to it. Genes control all three.
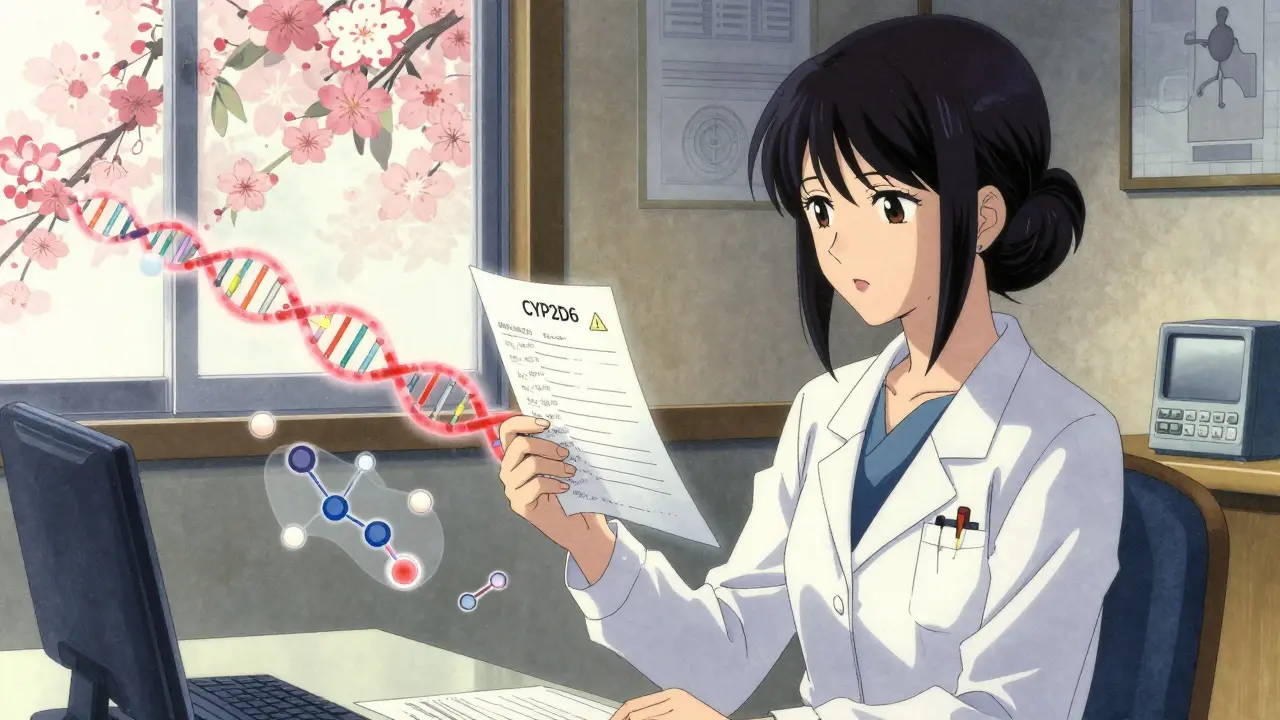
Take the cytochrome P450 enzymes - a family of proteins that do the heavy lifting in drug metabolism. Three of them - CYP2D6, CYP2C19, and CYP2C9 - are responsible for processing more than half of all commonly prescribed medications. But not everyone has the same version of these genes. Some people have variants that make these enzymes work too slowly (poor metabolizers), too quickly (ultrarapid metabolizers), or not at all.
For example, CYP2D6 turns codeine into morphine. If you’re an ultrarapid metabolizer, your body converts codeine so fast that it can build up toxic levels of morphine - even from a normal dose. The FDA has issued black box warnings for this exact scenario: nursing mothers who are ultrarapid metabolizers can unknowingly poison their babies through breast milk. In contrast, poor metabolizers get almost no pain relief from codeine because their bodies can’t convert it at all.
It’s not just about metabolism. Some genes affect the drug’s target - the exact spot in your body where the drug is supposed to act. If that target is slightly different because of your DNA, the drug might overstimulate it, under-stimulate it, or trigger an immune reaction instead.
High-Risk Gene-Drug Pairs You Should Know
The FDA maintains a living list of gene-drug interactions with clear clinical guidance. As of October 2023, it included 128 such pairs. A few stand out because the risks are severe and the genetic link is unmistakable.
- HLA-B*15:02 and carbamazepine/phenytoin: Carriers of this gene variant - common in Southeast Asian populations - face a 100 to 150 times higher risk of developing Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis (SJS/TEN). These are rare but deadly skin reactions that cause blistering, peeling skin, and organ failure. Screening for this variant before prescribing these seizure medications is now standard in many countries.
- CYP2C19 and proton pump inhibitors (PPIs): Poor metabolizers of CYP2C19 (about 2-5% of Europeans, up to 15% in East Asians) can have 5 to 10 times higher levels of drugs like pantoprazole. This increases side effects like low magnesium, bone fractures, and kidney damage. Dosing guidelines exist specifically for these patients.
- VKORC1 and CYP2C9 with warfarin: Warfarin, a blood thinner, has a narrow safety window. Too little, and you clot; too much, and you bleed. The VKORC1 -1639G>A variant explains 25-30% of dose variability. CYP2C9 variants add another 10-15%. Together, these genes can determine whether someone needs 1 mg or 7 mg per day. Without genetic testing, it can take weeks of trial and error to find the right dose - during which time patients are at high risk.
- SLCO1B1 and statins: A single variant in this gene increases the risk of statin-induced muscle pain by 4.5 times. This isn’t just discomfort - severe muscle damage can lead to kidney failure. Testing for this variant is now recommended before starting high-dose statins.
These aren’t edge cases. They’re predictable, preventable, and well-documented. Yet most people never get tested.

Why Some Side Effects Are More Predictable Than Others
Not all side effects are created equal when it comes to genetics. A 2024 study in PLOS Genetics analyzed over 10,000 drug reactions and found something surprising: side effects that mirror traits already linked to genetics are far more predictable.
Take cardiovascular reactions. If a drug causes high blood pressure or irregular heartbeat, and those same symptoms are known to run in families due to genetic factors, then the genetic link to the side effect is strong. The study found a 29.8% positive predictive value - meaning nearly one in three people with the right gene variant will experience the side effect. That’s high enough to justify testing.
On the flip side, nausea, diarrhea, or dizziness? Those are harder to predict genetically. Their predictive value is below 10%. Why? Because they’re often caused by unrelated factors - diet, stress, gut bacteria, or even how fast you took the pill.
This matters because it tells us where to focus. Instead of testing everyone for everything, we should target high-risk, high-impact scenarios: life-threatening reactions, drugs with narrow safety margins, and conditions where the genetic signal is clear.
Real-World Impact: When Testing Changes Outcomes
At Vanderbilt University’s PREDICT program, which started in 2011, doctors began routinely testing patients for key genetic variants before prescribing. The results? For 12.3% of patients, the test changed their treatment plan - usually by avoiding a drug or lowering the dose.
One woman with breast cancer was about to start tamoxifen. Her sister had suffered severe nausea and vomiting on the same drug. Genetic testing revealed she was a CYP2D6 poor metabolizer - meaning her body couldn’t activate tamoxifen properly. Her doctor switched her to an alternative, and she avoided the side effects entirely. "It took three weeks to get the results," she told a Reddit community. "But it saved me from what my sister went through."
Another case: a 7-year-old boy with epilepsy. His neurologist ordered HLA-B*15:02 testing before starting carbamazepine. The test came back positive. The drug was switched to valproate. He never had a reaction. Without that test, he might have developed SJS/TEN - a condition with a 30% death rate.
But here’s the catch: these stories are still rare. A 2023 survey of over 1,200 U.S. doctors found that 68.5% felt untrained to interpret genetic results. Only 22.3% used pharmacogenetic testing routinely. Most primary care providers still don’t know what CYP2D6 even means.

The Barriers: Cost, Access, and Confusion
Testing isn’t magic. It’s complicated. A full pharmacogenetic panel costs between $249 and $499. Insurance often doesn’t cover it unless you’ve already had a bad reaction. Medicare Advantage plans covered preemptive testing for only 28% of patients in 2023.
Even when the test is done, interpretation is messy. CYP2D6, for example, has over 100 known variants. Some are common. Others are structural - like gene duplications or deletions - and require expert review. About 15-20% of results need a pharmacogenetics specialist to decode.
Electronic health records rarely integrate test results meaningfully. A doctor might see "CYP2D6 Poor Metabolizer" on a report and have no idea what to do next. That’s why only 37% of U.S. hospitals have clinical decision support tools built into their systems.
And then there’s the data gap. Over 90% of pharmacogenetic studies are based on people of European ancestry. But African populations have 30% more genetic diversity. A variant common in West Africa might be completely absent in European databases - meaning someone could be misclassified, or missed entirely.
What’s Next? The Future of Personalized Prescribing
Things are changing. The FDA’s 2023 draft guidance proposes requiring pharmacogenetic data for 35+ new drugs by 2027. The All of Us Research Program has returned genetic results to over 215,000 Americans - and found that 42% carry at least one actionable variant.
Some hospitals are ahead of the curve. Mayo Clinic’s RIGHT Protocol has cut ADR-related hospitalizations by 23% using preemptive genotyping. St. Jude Children’s Research Hospital reduced pediatric ADRs by 33% after implementing routine testing.
But the biggest shift might come from whole-genome sequencing. A 2023 JAMA study showed that sequencing all 10 key pharmacogenes found actionable variants in 91% of participants. Over 10 years, this could prevent 1.2 adverse reactions per person.
Still, the real challenge isn’t science - it’s systems. We know which genes matter. We know which drugs are risky. We have the tools. What we lack is consistent implementation: training for doctors, integration into clinics, fair access across populations, and insurance coverage that matches the science.
For now, if you’ve had strange or severe side effects from a medication - especially if family members have too - ask your doctor: "Could this be genetic?" It’s not paranoia. It’s precision medicine.
Can genetic testing prevent drug side effects?
Yes, in specific cases. Genetic testing can prevent life-threatening reactions like Stevens-Johnson Syndrome from carbamazepine in HLA-B*15:02 carriers, or fatal overdoses from codeine in ultrarapid metabolizers. It can also help avoid ineffective treatments, like tamoxifen in CYP2D6 poor metabolizers. But testing doesn’t prevent all side effects - only those strongly linked to known genetic variants.
Which drugs require genetic testing before use?
The FDA recommends genetic testing before using 18 drugs, including warfarin (CYP2C9/VKORC1), abacavir (HLA-B*57:01), carbamazepine (HLA-B*15:02), clopidogrel (CYP2C19), and codeine (CYP2D6). Many others have guidelines but aren’t yet mandatory. Oncology drugs like irinotecan (UGT1A1) and tamoxifen (CYP2D6) are increasingly tested in clinical practice.
Is pharmacogenetic testing covered by insurance?
Coverage varies widely. Medicare covers only 7 of the 128 FDA-recognized gene-drug pairs. Most private insurers cover testing only after a side effect occurs, not before. Some plans cover preemptive testing for high-risk drugs like warfarin or clopidogrel. Out-of-pocket costs range from $249 to $499. Always check with your insurer before testing.
Can I get tested without a doctor’s order?
Yes, companies like 23andMe, Color Genomics, and OneOme offer pharmacogenetic panels directly to consumers. But be cautious: many of these tests aren’t validated for clinical use. The FDA has issued 12 warning letters to companies for overstating their results. A positive result from a direct-to-consumer test should always be confirmed and interpreted by a healthcare provider.
Why don’t all doctors use genetic testing for prescribing?
Three main reasons: lack of training, poor integration into electronic health records, and unclear reimbursement. A 2023 survey found 68.5% of doctors felt unprepared to interpret results. Many hospitals don’t have clinical decision support tools. And without insurance coverage, testing is often seen as a luxury, not a necessity - especially in primary care.




lol so now we're gonna test everyone's DNA before giving them ibuprofen? next they'll be scanning your iris to see if you're 'compatible' with aspirin. this is what happens when science gets a TED Talk and a VC funding round. i took naproxen once and felt like a ghost. turns out i'm just a ghost. not a poor metabolizer. just bad luck. 🤷♂️
The clinical utility of pharmacogenomic testing is not theoretical-it's evidenced. CYP2D6 ultrarapid metabolizers on codeine present with life-threatening morphine toxicity; HLA-B*15:02 carriers on carbamazepine have a >100x risk of SJS/TEN. These are not 'edge cases'-they're iatrogenic catastrophes waiting to happen. The barrier isn't science; it's infrastructure. EHRs lack decision support, providers lack training, and payers lack incentive. We need embedded CDS, mandatory CME modules, and reimbursement parity. This isn't precision medicine-it's damage control.
This is actually one of the most important topics in modern medicine 😊 I had no idea my mom's bad reaction to warfarin was genetic until I read this. She’s been on a stable dose for 8 years now, and we finally know why it took 6 months to get it right. I just got my 23andMe results back and am scheduling a pharmacist consult. If this helps even one person avoid hospitalization? Worth it. 🙌
Wait... so you're telling me the government, Big Pharma, and the FDA are ALL hiding this? 🤔 They've known about CYP2D6 variants since the 90s... but they don't test? And insurance won't cover it? Hmmm... sounds like a classic cover-up. They don't want you to know your body can 'reject' drugs naturally-because then you'd stop taking their $500 pills and start asking questions. I read a blog once that said 97% of drug side effects are caused by electromagnetic fields from cell phones... maybe that's why they don't test? 🤫
I've been thinking about this nonstop since I read this. My cousin died from a reaction to an antibiotic in 2017. Autopsy said 'idiosyncratic'. But what if it wasn't? What if her CYP3A4 was a slow metabolizer and they just didn't test? I looked up her 23andMe raw data. Found a variant in SLCO1B1. Called her doctor. They said 'we don't do that here'. So now I'm crowdfunding a genetic panel for my whole family. Because if we don't, who will? My sister's on statins. My nephew's on ADHD meds. My mom's on blood pressure pills. We're all walking time bombs. And nobody's telling us. I just want to know if I'm gonna die because of a gene I never knew I had.
I don't know why this feels so heavy. Like, I'm sitting here reading about people poisoning their babies from codeine, and I'm just... quiet. I took oxycodone after surgery last year. Felt fine. But what if I was one of those ultrarapid metabolizers? What if I'd been the one who passed out in the ER? It's terrifying how much of your body you don't control. The science is real. The stakes are real. But the system? It's still stuck in the 1980s. We're using DNA to predict crime and credit scores, but not to keep people alive? Weird.
THIS IS WHY WE NEED TO STOP TRUSTING DOCTORS. They don't even know what CYP2D6 means. You think your GP reads journals? No. They get a 10-minute slide deck from a pharma rep who says 'this drug is safe'. Meanwhile, your kid's skin is peeling off because they didn't test for HLA-B*15:02. And you're supposed to be grateful? No. We need to boycott all drugs until every prescriber is forced to take a genetics course. And if they refuse? Fire them. This isn't medicine. It's Russian roulette with a prescription pad.
I'm from Australia and we've had pharmacogenomic testing integrated into our PBS (Pharmaceutical Benefits Scheme) for warfarin and clopidogrel since 2018. It's not perfect, but we've cut ADRs by 30% in high-risk groups. The key? Government funding, standardized guidelines, and training for GPs. We didn't wait for EHRs to catch up-we built workflows around the data. It's not magic. It's policy. America's problem isn't science. It's capitalism. You can't monetize 'prevention' if it doesn't involve a new drug. So they don't. But here? We do. And people live longer.
so like... if i'm a slow metabolizer and i take a drug that's supposed to be activated by my liver... does that mean i'm basically just a zombie? like i take the pill and nothing happens? am i just... not real? 🤔
I work in a clinic and we started offering free CYP2D6 testing last year. One woman came in because her sister died on codeine. She was about to get tamoxifen. Test came back: poor metabolizer. We switched her to anastrozole. She cried. Said she felt like someone finally saw her. That’s the moment. Not the science. Not the data. The human. We need more of this. Not less.