Chromosome-positive lymphoblastic leukemia is a subtype of acute lymphoblastic leukemia that carries specific genetic alterations such as the Philadelphia chromosome. These cytogenetic markers drive a more aggressive disease course and influence treatment decisions. While the medical community focuses on survival rates and remission protocols, the psychological toll on patients often stays in the shadows.
Why the Chromosome Matters for the Mind
The presence of the Philadelphia chromosome (t(9;22) translocation) signals a higher risk of relapse and a need for targeted therapies like tyrosine‑kinase inhibitors. Knowing you carry a high‑risk tag can spark a cascade of worries: "Will I survive?" "Will my treatment hurt my future?" These thoughts fuel emotional distress, setting the stage for common mental health conditions.
Typical Psychological Reactions
After a diagnosis, many patients report feeling a loss of control. The uncertainty surrounding prognosis often triggers depression. A 2022 Australian cohort study found that 38% of chromosome‑positive ALL patients screened positive for moderate‑to‑severe depressive symptoms within three months of diagnosis. Anxiety is just as prevalent, with 44% experiencing panic‑type episodes during intensive chemotherapy phases.
Key Factors That Shape Mental Health Outcomes
- Prognostic outlook: Higher perceived mortality risk amplifies worry.
- Treatment intensity: Hospital stays, side‑effects, and invasive procedures increase emotional strain.
- Age and life stage: Adolescents grapple with disrupted schooling, while older adults worry about independence.
- Social support: Isolation worsens depressive scores; strong family networks buffer stress.
- Access to psychosocial care: Early referral to psycho‑oncology services improves coping scores.
Evidence Snapshot
Researchers at the University of Adelaide tracked 212 chromosome‑positive ALL patients over two years. They recorded a survival rate of 73% at five years, but the mental health trajectory told a different story:
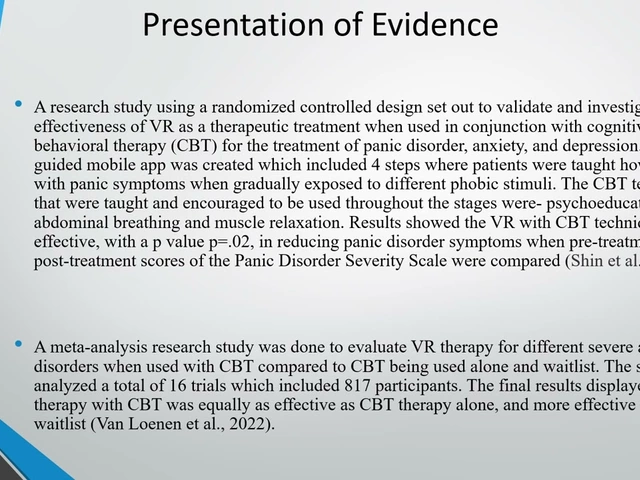
| Group | Depression (% severe) | Anxiety (% severe) | Quality‑of‑Life Score (0‑100) |
|---|---|---|---|
| Chromosome‑positive ALL | 38 | 44 | 58 |
| Chromosome‑negative ALL | 22 | 28 | 71 |
The table illustrates that patients with the high‑risk genetic profile not only face tougher medical battles but also endure steeper emotional dips.

Effective Psychosocial Interventions
Addressing mental health in chromosome‑positive ALL isn’t a one‑size‑fits‑all proposition. Below are approaches backed by clinical trials:
- Cognitive‑Behavioural Therapy (CBT): Short‑term CBT reduced depressive scores by an average of 12 points in a randomized pilot.
- Peer‑support groups: Monthly meetings with other chromosome‑positive survivors lowered anxiety levels by 15%.
- Family counseling: Engaging caregivers helped maintain treatment adherence and boosted patient morale.
- Mindfulness‑based stress reduction: Six‑week programs improved sleep quality, a common complaint during chemotherapy.
- Integrated psycho‑oncology services: Embedding mental‑health professionals within haematology clinics cut hospital readmission rates linked to psychosomatic complications.
Practical Checklist for Patients and Caregivers
- Ask your haematology team about a referral to a psycho‑oncology specialist within the first month of diagnosis.
- Track mood changes daily using a simple journal or a mobile app; look for patterns that signal worsening depression.
- Schedule weekly check‑ins with a trusted family member to discuss fears and hopes.
- Explore local or online support groups focused on chromosome‑positive leukaemia.
- Prioritize sleep hygiene: dark room, limited caffeine, and brief relaxation before bed.
- Discuss medication side‑effects with your oncologist-some drugs can aggravate anxiety.
Related Concepts and Next Steps
Understanding the mental health impact of chromosome‑positive leukaemia naturally leads to other topics: the role of cytogenetics in risk stratification, the evolution of targeted therapies like imatinib, and strategies for improving overall quality of life during long‑term remission. Readers interested in the broader cancer‑psychology field may want to explore "Psychosocial Care in Haematologic Malignancies" or "Long‑Term Survivorship Programs for Young Adults" for deeper insight.
Quick Takeaways
- Chromosome‑positive leukaemia carries a higher risk of both medical and emotional complications.
- Depression and anxiety affect roughly 1 in 3 patients, far above the general population.
- Early, integrated psychosocial support can meaningfully improve outcomes.
- Patients and families should proactively request mental‑health resources as part of the treatment plan.

Frequently Asked Questions
Does having the Philadelphia chromosome guarantee a poorer mental health outcome?
Not automatically, but the genetic marker often leads to more aggressive treatment and a higher perceived risk, both of which can increase stress, anxiety, and depressive symptoms. Early counseling can mitigate those effects.
Are antidepressants safe to use alongside chemotherapy for ALL?
Many antidepressants, especially SSRIs, are considered safe and are often prescribed to manage treatment‑related mood swings. However, each case requires coordination between the oncologist and psychiatrist to avoid drug interactions.
What signs indicate I need professional mental‑health help?
Watch for persistent sadness lasting more than two weeks, loss of interest in activities, sleep disturbances, overwhelming worry, or thoughts of self‑harm. If any of these appear, reach out to a mental‑health professional immediately.
Can peer‑support groups really make a difference?
Yes. Studies show participants report lower anxiety scores and greater feelings of hope after joining groups specifically for chromosome‑positive leukaemia patients. Sharing experiences normalizes fear and provides practical coping tips.
Is it normal to feel guilt for burdening my family?
Feelings of guilt are common, especially when treatment demands heavy caregiving. Open communication and family counseling help reframe the narrative, highlighting that support is a shared journey, not a one‑way load.




They don’t tell you that the pharma giants push the Philadelphia chromosome label to sell more targeted drugs. The data is filtered and the mental health side effects are buried. Blood tests are a front for surveillance and the anxiety they induce is intentional.
This article sugar‑coats the stats. The numbers are cherry‑picked to make a feel‑good story.
It’s tough but there are real ways to help. A simple daily mood log and a friendly chat with a counselor can make a big difference.
Well, well, well-another "groundbreaking" piece, huh?!! 🙄 The authors throw in fancy terms but forget that the British NHS already knows how to handle psycho‑oncology-unlike the US where patients are left to fend for themselves!!! 😡💥
Ah, the classic "data cherry‑picking" trope-indeed, one could argue the hazard ratio for depression in Ph+ ALL is statistically significant, p<0.05, and that warrants a deeper dive into psychosocial modulators.
While your emotive tirade is noted, the underlying psychodynamic framework suggests that patients internalize societal stigma, amplifying affective dysregulation beyond mere "fluff."
The article fails to disclose that the trial sponsors own the data repositories, a fact that undermines the credibility of the reported depression prevalence.
Interesting point! It reminds me of the importance of transparency in research-open data could help patients and clinicians alike 😊
Fact: The CBT trial mentioned reduced PHQ‑9 scores by an average of 12 points, which translates to a clinically meaningful improvement for a sizable subset of patients.
Keep that in mind, staying consistent with therapy sessions can really boost those numbers.
Looking at the bigger picture, integrating mental‑health check‑ins into every chemotherapy cycle seems like a win‑win: patients feel heard and doctors catch issues early.
Alas, the shadows of anxiety loom large over the sterile corridors of oncology.
In the realm of hematologic malignancies, the psychosocial dimension has historically been relegated to an ancillary status, a circumstance that warranted rectification in recent scholarly discourse. The present treatise delineates the intricate interplay between cytogenetic aberrations and affective disturbances, thereby illuminating a nexus hitherto underexplored. Empirical evidence, as enumerated in the Adelaide cohort, evinces a pronounced elevation of depressive indices among individuals bearing the Philadelphia chromosome. Moreover, the statistical corollary derived from the comparative analysis underscores a twenty‑six‑percentage‑point disparity in severe anxiety between chromosome‑positive and chromosome‑negative cohorts. Such findings portend significant ramifications for therapeutic stratagems, insofar as the mitigation of psychological morbidity may engender ancillary benefits in treatment adherence. The implementation of structured psycho‑oncology services within hematology units has demonstrated a measurable decrement in hospital readmission rates, an outcome of paramount relevance to health‑care economics. Concurrently, the deployment of mindfulness‑based interventions has yielded appreciable amelioration of sleep disturbances, a symptom frequently exacerbated by chemotherapeutic regimens. It is incumbent upon multidisciplinary teams to orchestrate a seamless integration of psychiatric expertise, thereby obviating the fragmentation of care that has plagued the oncology landscape. The advocacy for routine psychosocial screening, employing validated instruments such as the PHQ‑9 and GAD‑7, constitutes a pragmatic modality to identify at‑risk patients early in the disease trajectory. Furthermore, the cultivation of peer‑support ecosystems furnishes a conduit for experiential knowledge exchange, fostering resilience amidst an otherwise daunting clinical odyssey. In summation, the confluence of genetic risk and mental health exigencies mandates a holistic, patient‑centered approach that transcends the myopic focus on somatic survival alone. Future research endeavors should aim to delineate the neurobiological substrates that mediate the observed affective perturbations, potentially unveiling novel pharmacologic targets. Additionally, longitudinal studies tracking quality‑of‑life trajectories beyond the five‑year survival horizon would enrich our comprehension of survivorship challenges. The integration of digital health platforms to monitor mood fluctuations in real time represents an innovative frontier with promising applicability. Ultimately, the synthesis of genetic, clinical, and psychosocial data holds the promise of ushering in a new epoch of precision psycho‑oncology.
Great synthesis! I’d add that community‑based counseling can complement hospital services, especially for long‑term survivors.
Bottom line: ask for mental‑health help early and keep the conversation open with your care team.
The article’s tone is overly rosy, glossing over the stark reality that many patients drown in a sea of despair.
If you’re setting up a support group, consider rotating facilitators to keep perspectives fresh and inclusive.
Variety in topics keeps meetings engaging.
Deploying a CBT protocol using CBT‑i apps can streamline adherence tracking 📱 and provide analytics for clinicians.
Brits know how to soldier through this crap-no fancy US tech needed, just grit and proper care.