The Federal Circuit Court doesn’t just hear appeals-it decides when generic drugs can hit the market, how long brand-name drug companies can protect their patents, and where lawsuits can be filed across the entire United States. This single court controls the fate of billions in drug sales, not because it’s the most powerful court in the country, but because it’s the only one that hears all patent cases. And when it comes to pharmaceuticals, its rulings shape everything from drug prices to patient access.
Why the Federal Circuit Rules on Every Patent Case
In 1982, Congress created the U.S. Court of Appeals for the Federal Circuit to fix a mess. Before then, patent cases were scattered across 12 regional courts. One district court might say a drug patent was valid; another might say it wasn’t. Companies couldn’t plan. Investors didn’t know what to expect. So Congress gave one court exclusive power over all patent appeals. That includes every single pharmaceutical patent case in the country.
That means if a generic drug maker files an Abbreviated New Drug Application (ANDA) to sell a cheaper version of a brand drug, and the brand company sues for patent infringement, the case starts in a district court-but it ends in the Federal Circuit. No matter where the case was filed. No matter which state the generic company is based in. The Federal Circuit has the final word.
ANDA Filings and the Nationwide Jurisdiction Rule
Before 2016, brand-name drug companies could only sue generic makers in places where they did business. If a generic company was based in Texas, the lawsuit usually stayed in Texas. But in March 2016, the Federal Circuit changed that forever in the case involving Mylan.
The court ruled that filing an ANDA with the FDA is enough to create legal jurisdiction anywhere in the U.S. Why? Because the FDA application itself proves the company intends to sell the drug nationwide. That means a generic company in California can be sued in Delaware-even if it has no offices, employees, or sales there.
The result? Delaware became the most popular place to file pharmaceutical patent lawsuits. Between 2017 and 2023, 68% of all ANDA cases were filed there, up from just 42% in the decade before. Why Delaware? Because its courts are experienced with patent law and move quickly. Brand companies love it. Generic companies hate it-it forces them to defend lawsuits in places they’ve never operated.
The Orange Book and the Rules for Listing Patents
The FDA doesn’t just approve drugs-it also keeps a public list called the Orange Book. This is where brand companies list patents they claim cover their drugs. If a patent isn’t listed, a generic company can skip the lawsuit and launch its drug faster.
In December 2024, the Federal Circuit made it crystal clear: you can’t list a patent just because it’s related to the drug. It has to actually claim the drug itself. In the case of Teva vs. Amneal, the court said a patent that only described a method of manufacturing the drug, not the drug’s use or composition, couldn’t stay on the list. That forced companies to be much more precise. Now, legal teams spend extra time mapping each patent to the exact drug formulation to avoid having it removed.
This ruling saved generic companies months of legal delays. It also made patent portfolios cleaner. Companies can no longer use “patent thickets”-filing dozens of weak patents just to block competition.

Why Dosing Patents Are Harder to Get
One of the biggest battlegrounds in pharma patent law is dosing. Can you patent a new way to take a drug? Like taking 10mg once a day instead of 5mg twice a day?
In April 2025, the Federal Circuit said no-not unless you prove something new. In the ImmunoGen case, the court found that simply changing the dose of a known cancer drug wasn’t enough to make it patentable. Both sides admitted the drug itself was already known. The only difference was how often it was given. The court said: if a skilled scientist could reasonably expect the same results with the new dose, then it’s obvious-and not patentable.
This was a major shift. In Europe, patent offices often grant dosing patents. In the U.S., the Federal Circuit now treats them like minor tweaks. Since that decision, pharmaceutical companies have cut back on filing dosing patents by 37%, according to Clarivate. Instead, they’re investing more in entirely new drug compounds.
Standing: Can You Challenge a Patent Before You Even Start?
Here’s a problem generic companies face: how do you challenge a patent before spending millions on clinical trials? You can’t just say, “I might make this drug someday.” The Federal Circuit says you need proof you’re actually doing it.
In May 2025, in the Incyte case, the court said you need concrete plans-like Phase I clinical trial data, manufacturing plans, or FDA communication. Without that, you don’t have “standing.” You can’t sue.
This has created a catch-22. To get standing, you need to invest money. But if you invest money, you risk being sued for infringement. Some companies now delay development just to avoid triggering litigation. Others are filing lawsuits earlier, even before they’ve fully designed the drug.
Some lawmakers are noticing. In early 2025, Senators Tillis and Coons introduced the Patent Quality Act, aiming to lower the bar for standing in pharmaceutical cases. They argue the current rule stifles competition. The Federal Circuit, for now, stands by its decision.

How This Affects Real Patients and Drug Prices
These legal rules don’t stay in courtrooms. They ripple through pharmacies, hospitals, and patients’ wallets.
Because the Federal Circuit makes it harder to challenge patents early, the average cost of an ANDA lawsuit jumped from $5.2 million to $8.7 million between 2016 and 2023. Those costs get passed on. Brand companies use the threat of litigation to delay generics longer. The 30-month stay on generic approval is now almost always used up-meaning patients wait years longer for cheaper versions.
On the flip side, when the court strikes down weak patents-like the dosing patents or improperly listed ones-it opens the door for generics to enter faster. That’s when prices drop. A single generic drug can cut the cost of a treatment by 80% or more.
The court’s decisions also affect biologics-complex drugs like those for rheumatoid arthritis or cancer. The same jurisdiction rules that apply to pills now apply to injectables. Biosimilar litigation has grown 300% since 2020. The Federal Circuit is now the gatekeeper for the next generation of affordable biologics.
Is the Court Too Powerful?
The Federal Circuit has built a reputation for being predictable-but also insular. It hears only patent cases. Its judges become specialists. That’s good for consistency. But critics say it’s too detached from real-world consequences.
For example, it reverses district court rulings in pharmaceutical cases at a rate of 38.7%, far higher than its overall patent reversal rate of 22.3%. That means district courts often get it wrong-sometimes because they don’t understand the science behind the drug.
There’s also the standing issue. Judge Hughes, in his concurrence in the Incyte case, openly worried that the court’s rules might be blocking competition. He said companies developing generic drugs have a real interest in challenging patents before they spend millions. But the court says that’s not enough.
Some patent attorneys say the court’s rulings have made the system clearer. Others say it’s become a barrier. The American Bar Association survey found 57% of practitioners think the dosing patent standards are too rigid. But 33% say they’re just right.
There’s no perfect answer. The court balances innovation against access. And right now, it’s leaning toward limiting patents on small changes.
What This Means for the Future
The Federal Circuit isn’t slowing down. Its rulings in 2024 and 2025 have already changed how companies file patents, where they sue, and when they launch drugs.
By 2027, analysts predict a 15-20% drop in “evergreening”-the practice of extending patent life with minor changes. Core drug patents will still hold strong (82% are still upheld). But the days of patenting a new pill schedule or a slightly different tablet coating are fading.
Generic companies are adapting. They’re filing lawsuits earlier. They’re documenting development plans more carefully. They’re working with legal teams who understand the Federal Circuit’s patterns.
Brand companies are tightening their patent lists. They’re focusing on truly novel inventions. And they’re preparing for more challenges.
The Federal Circuit doesn’t make drugs. It doesn’t prescribe them. But it decides who can make them-and when. And in a system where patents control access to medicine, that’s power.
What is the Federal Circuit Court’s role in pharmaceutical patent cases?
The U.S. Court of Appeals for the Federal Circuit is the only appellate court in the United States that hears all patent appeals, including those involving pharmaceutical drugs. It has exclusive jurisdiction over cases involving patent validity, infringement, ANDA litigation, and disputes over the FDA’s Orange Book listings. Its decisions set binding legal standards for the entire country.
Why does the Federal Circuit have jurisdiction over cases filed anywhere in the U.S.?
In 2016, the court ruled that filing an Abbreviated New Drug Application (ANDA) with the FDA shows intent to market a generic drug nationwide. That single act creates personal jurisdiction in any U.S. district court, even if the generic company has no physical presence there. This allows brand-name companies to choose favorable venues like Delaware.
Can you patent a new dosage regimen for an existing drug?
It’s very difficult. In 2025, the Federal Circuit ruled that changing the dose, frequency, or schedule of a known drug is not patentable unless the applicant proves unexpected results. Simply showing a new dosing pattern isn’t enough-you must show the change leads to a surprising improvement, like fewer side effects or better efficacy that couldn’t be predicted from prior art.
What is the Orange Book, and why does it matter?
The Orange Book is the FDA’s official list of approved drug products and their associated patents. Brand companies must list patents that claim the drug’s chemical structure, formulation, or method of use. In 2024, the Federal Circuit ruled that patents not directly claiming the drug must be removed. This prevents companies from blocking generics with irrelevant patents.
Do generic drug makers need to have started development to challenge a patent?
Yes. Since the 2025 Incyte decision, generic companies must show concrete evidence of development-like Phase I clinical trial data, manufacturing plans, or FDA correspondence-to have legal standing to challenge a patent. Simply intending to make the drug isn’t enough. This has forced companies to document their plans earlier and more thoroughly.
How do Federal Circuit rulings affect drug prices?
The court’s rulings directly impact when generics enter the market. When it upholds weak patents, it delays competition and keeps prices high. When it strikes down patents-like improper Orange Book listings or obvious dosing claims-it allows generics to launch sooner, often cutting prices by 80% or more. The court’s strict standards are helping reduce “evergreening,” which should lead to lower prices over time.

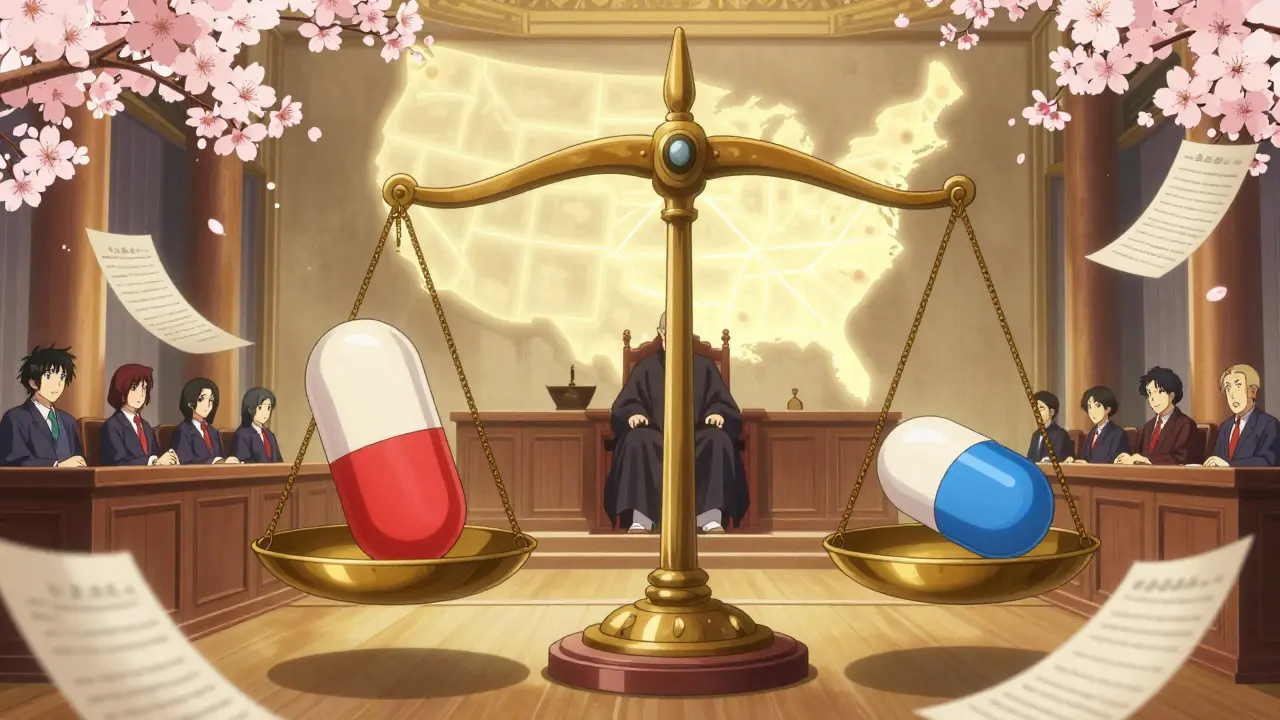



So let me get this straight - a bunch of judges in D.C. who’ve never seen a pill in their life get to decide if a $200 drug stays expensive or drops to $5? And we call this justice? 🤡
Meanwhile, in India, generics save lives daily. But here? You need a PhD in patent law just to breathe without getting sued.
There’s real value in having one court handle all patent cases - consistency matters, especially when you’re talking about life-saving meds.
Yes, Delaware got weirdly popular, but that’s because they’ve built real expertise. The alternative? 12 different courts with 12 different standards. That’s chaos.
The dosing patent ruling? Long overdue. Patents shouldn’t be loopholes for lazy pharma. Innovation should mean new molecules, not new pill schedules.
Let’s be real - this court isn’t broken, it’s American. We built the best pharmaceutical system in the world, and now some woke generic companies want to tear it down because they can’t be first?
The Federal Circuit protects American innovation. If you can’t afford to fight a patent lawsuit in Delaware, maybe you shouldn’t be in pharma.
Also, India? Please. They copy everything. We don’t need their ‘cheap drug’ mentality here.
Ohhh so the Federal Circuit is the Oracle of Patents now? The High Priestess of Pill Politics?
They don’t make drugs - they make *myths*. The myth that a patent is a divine right. The myth that ‘innovation’ means stretching a molecule’s shadow for 20 more years.
Meanwhile, patients are paying $1000 for insulin because a judge decided ‘once daily’ is somehow a ‘novel invention.’
Is this law? Or is this a Shakespearean tragedy where the only tragedy is that we still believe in it?
I think the court’s getting it right - especially with the Orange Book cleanup. Too many companies were just dumping every patent they had in there like a closet full of old socks.
And the dosing rule? Honestly, it makes sense. If you’re just changing when you take it, that’s not a new drug - that’s just… better instructions.
Hope this means more affordable meds soon 💛
Delaware being the hotspot for these lawsuits is wild but kinda makes sense
They’ve got the judges who get it and move fast
Generic companies are stuck between a rock and a hard place though
Have to prove you’re gonna make the drug to sue but making the drug means you get sued
Feels like a trap
Big win for patients with the dosing patent ruling
Too many companies were just gaming the system with tiny tweaks
Real innovation should be rewarded not lazy patent padding
Also the Orange Book fix? Yes please
Less noise more truth
YESSSS this is exactly what we need 😍
Patents shouldn't be used to block competition like a toddler hugging a toy
Generic drugs = more access = better health for everyone 💪💊
Thank you Federal Circuit for finally getting it right!!! 🙌
the court is too powerful and no one talks about it
one court controls all patents
what if they get it wrong
what if they dont understand the science
patients pay the price
its not fair
There’s something poetic about a court that never sees a patient but decides who gets medicine
It’s like a god of bureaucracy deciding who breathes and who doesn’t
Patents were meant to encourage innovation, not create monopolies disguised as IP
Maybe we need to ask - is this law… or is this capitalism with a robe on?
Wow. Just wow. You people act like this is some heroic reform. It’s not. It’s damage control.
You think this court is fair? They reversed district courts 38% of the time in pharma cases - that means district judges are drowning in complexity.
And now you want to celebrate them? They’re not heroes. They’re the last stop on a broken train.
Stop pretending this is justice. It’s legal roulette with your life on the line.
kinda surprised they actually stuck to the science on the dosing thing
used to be you could patent breathing if you did it at night
glad they’re cutting the fat
still wish they’d fix standing rules tho
generic companies are stuck waiting for permission to even try
As someone from India where generics are lifelines, I see this clearly: the Federal Circuit is the gatekeeper of global access.
When they strike down weak patents, it ripples across the world - cheaper drugs for Africa, Asia, Latin America.
But when they protect evergreening, it’s not just American patients who suffer - it’s the entire global health ecosystem.
Delaware’s dominance isn’t legal efficiency - it’s economic colonialism.
And yes, dosing patents being rejected? Long overdue. Science doesn’t reward obviousness.
Let innovation breathe - not patent lawyers.